What are polar surfaces?
When you think of charged surfaces, you might imagine acid-base chemistry where protons are transferred between the functional groups at the surface and the solution. Or you might think of an electrode that’s held at constant electropotential. You might even consider a combination of the two. Maybe less obvious is that surface charge can originate from the structure of the crystal itself.
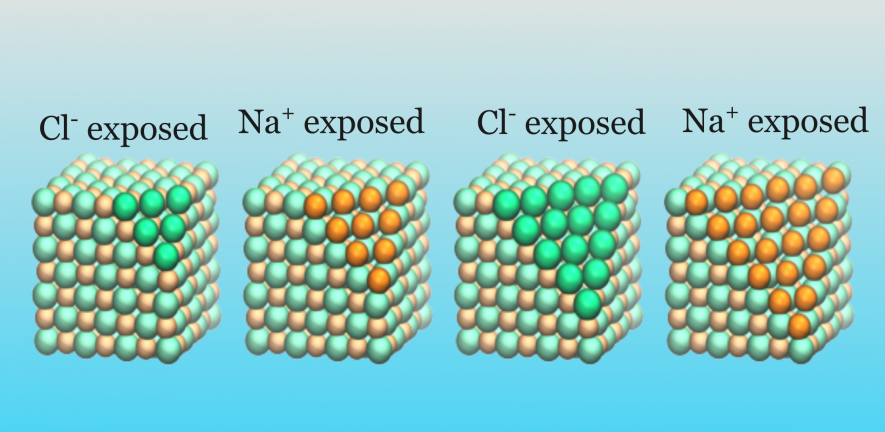
Simple table salt provides an everyday example. Imagine that we create surfaces by successively removing a layer of ions, starting from the corner of the crystal, like we show in the picture. Each time, the exposed surface is charged, containing just Na+ (orange) or Cl- (green) ions.
Why do we care?
We are interested in these polar surfaces because they are catalytically active, can control the shapes of crystals (at both small and large length scales) and influence how particles in solution interact with each other. We also think their physical chemistry is fun!
Our work
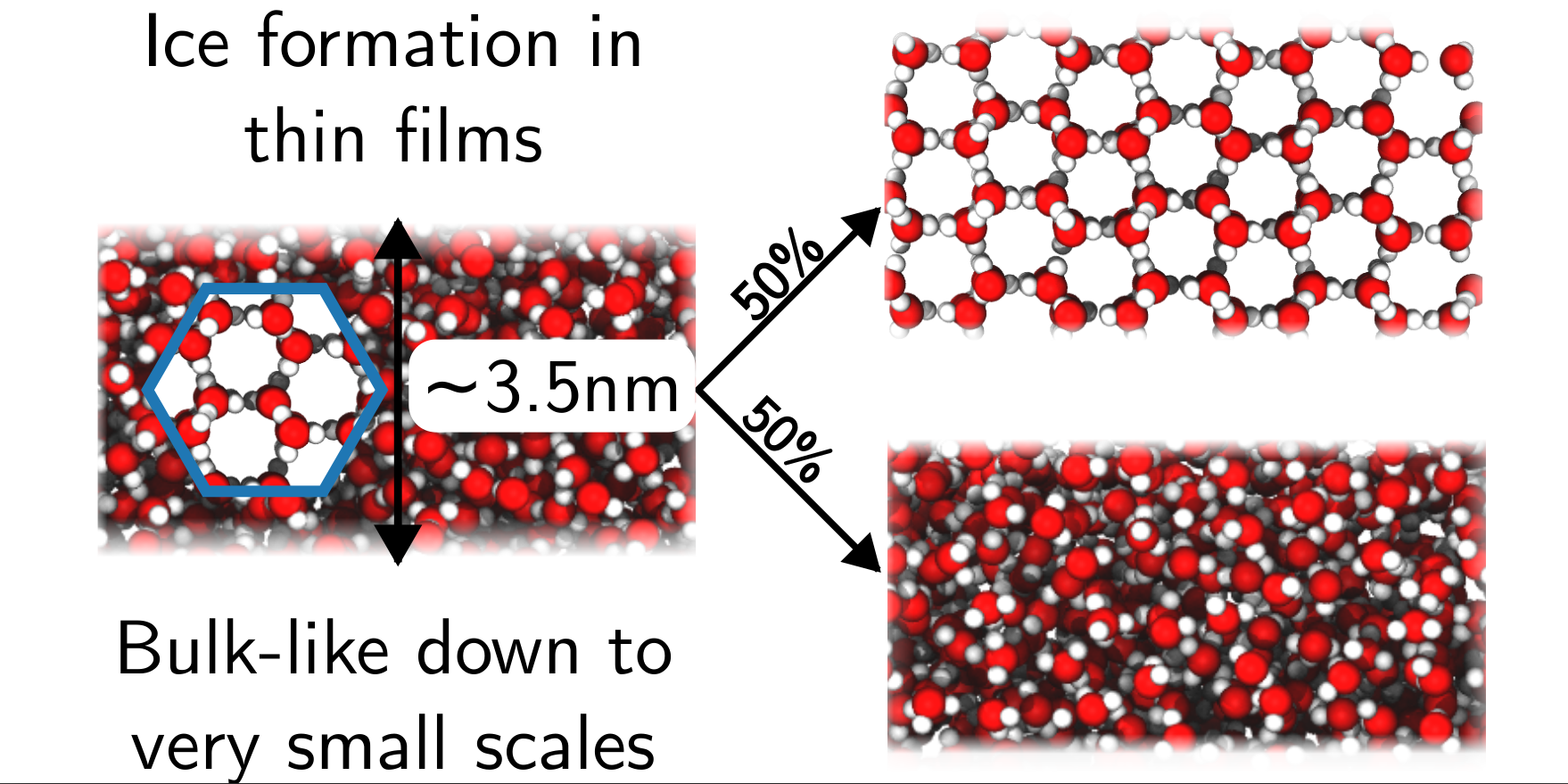
We have had to overcome many technical challenges when trying to simulate polar surfaces in contact with liquid solutions. We have found that standard simulation methods lead to qualitatively incorrect results whereby dissolved ions are repelled from charged crystal surfaces.
We have shown how a computational technique (the “finite field approach”) can be used to approximate the surfaces of macroscopic polar crystals with microscopic simulations. We have also developed a theoretical framework to understand polar crystal surfaces in solution.
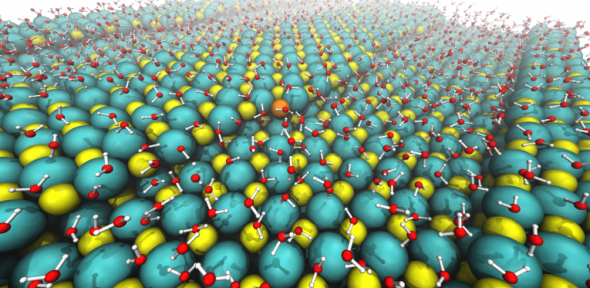
We have applied our understanding to heterogeneous ice nucleation at AgI. There is a long-held belief that AgI helps water freeze because its structure is similar to ice. We found that for this “template effect” to hold, dissolved ions are essential. These dissolved ions also play a key role in the ice nucleation mechanism.
Future Challenges
We know that the solution environment can act to stabilize polar surfaces… but does it?! We also know that under “clean conditions,” polar surfaces can reconstruct. So, we now want to further develop the computational frameworks needed to understand what crystal surfaces look like in solution. We aim to understand how we can control surface structure, e.g., by introducing additives into solution.
We also care about how crystals form. By manipulating conditions, experimental research groups can control the shapes of crystals that form. This includes exposing polar surfaces. We aim to use molecular simulations to provide insight—at the level of individual molecules—into what controls crystal formation in solution.