
Research Associate
I joined the Bender research group as Research Associate in 2019 to work on a project funded by Unilever aiming at defining adversity and adaptation tipping points. The effect of chemical stressor on a cell can be considered as a function of both dose and time [1]. At low doses and short duration of exposure, any perturbation to normal cell function is within a cell’s innate capacity to repair by using posttranslational mechanisms (capacity-limited antioxidant activity e.g., using cell reserves of glutathione). Transcriptional stress control is unlikely to be sufficient for robust adaptation, due primarily to the time required to go from transcriptional activation to completed synthesis of new gene products. As dose or duration of exposure increases, the cell’s innate repair capacity is diminished. When a tipping point is reached, activation of transcription factors occurs, leading to the transcription of genes that are required to respond to the stress. This change in cell state may be transient, once the stressor is removed and the damage-repaired cell returns to its original state, or it may be permanent, leading to a permanently (pathologically) altered cell state. When this occurs, the cell may become incapable of carrying out its specialized function within a tissue. At very high doses, cell death may occur.
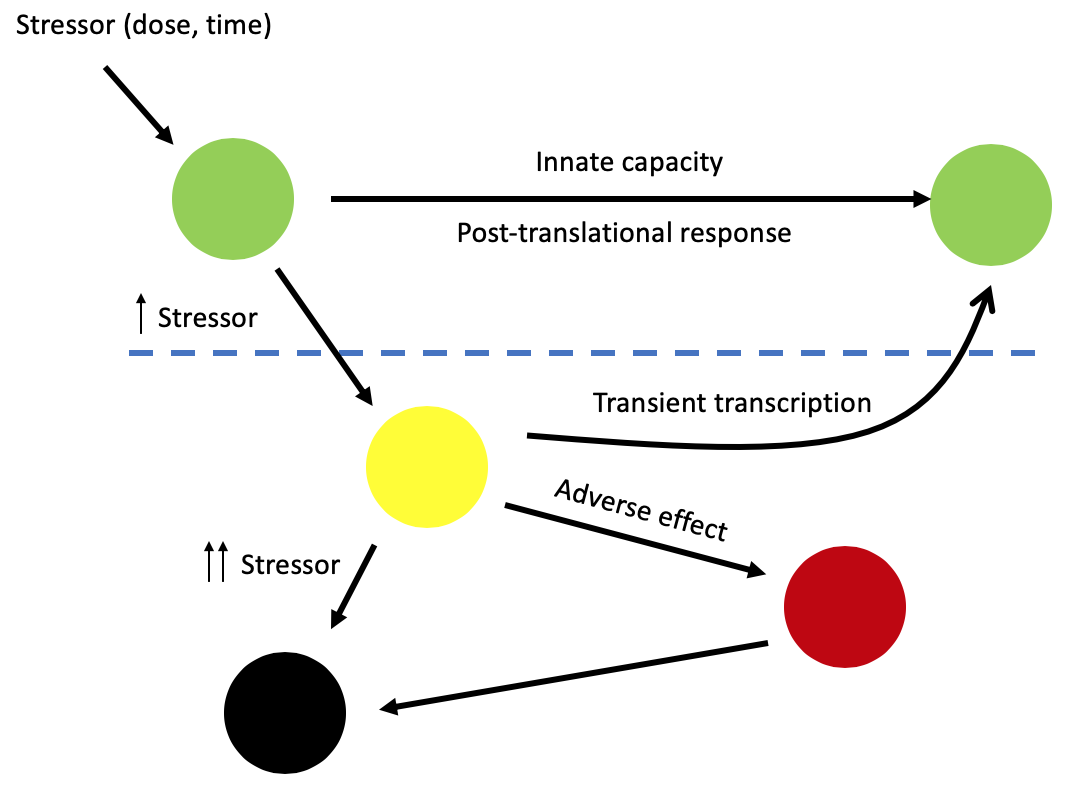
The stress level at which posttranslational control reaches a maximum may correspond to a threshold above which the controlled cellular state significantly deviates from the baseline level, leading to transcriptional activation of a broad suite of gene products, including both increased levels of stress proteins and other cellular function pathway proteins. This threshold may serve as a point of departure where cells transition from adaptation to stressed and to stress-related adversity. Most of the work in the scientific community has focused on modelling the dose-response characteristics present in gene expression data to derive the NOTEL, the lowest dose of a compound that might trigger a transcriptional response in cells. However, using the NOTEL to set low-risk exposure levels may not be the correct approach as it seeks to preclude all transcriptional activity even though we may not have the biological knowledge to determine which level of gene expression perturbation is adverse. In this context, my research focuses on leveraging gene expression data to develop system biology approaches able to discriminate between adaptive and adverse molecular responses and to define a NOTEL to set low-risk exposure levels not biased by adaptive responses.
Thanks to Unilever for funding this project.
REFERENCES
1. https://www.liebertpub.com/doi/10.1089/aivt.2017.0003
2. https://academic.oup.com/toxsci/article/147/2/302/1620690
Publications
- <
- 2 of 2
