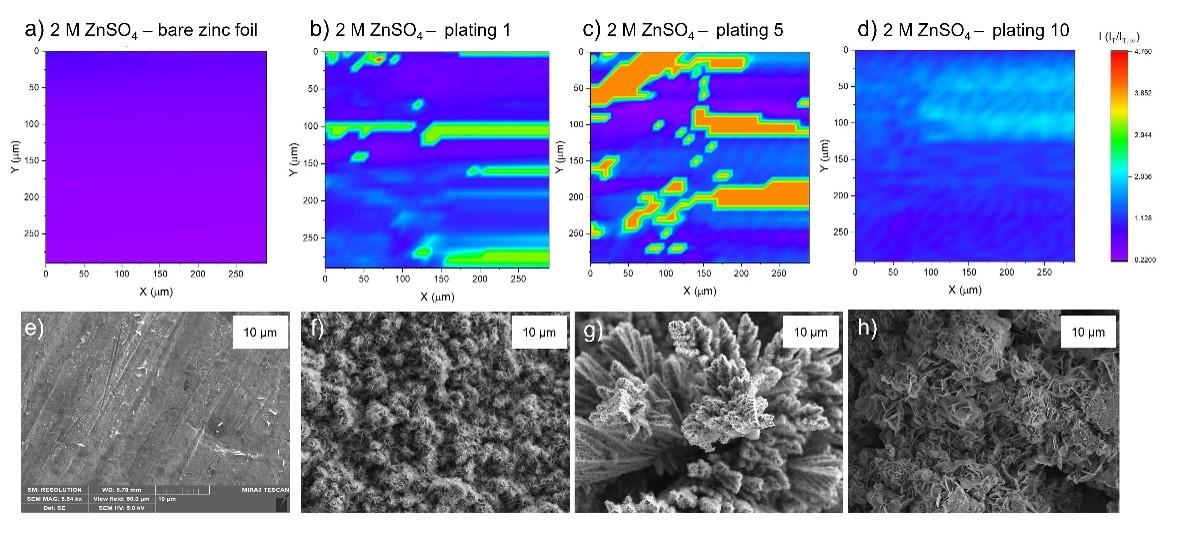
The effect of interface heterogeneity on zinc metal anode cyclability
J. T. Simon, V. ˇSedajova, D. Tripathy, H. E. Smith, S. M. Clarke, C. P. Grey and S. Menkin
Zinc metal batteries (ZMBs) are promising candidates for low-cost, intrinsically safe, and environmentally
friendly energy storage systems. However, the anode is plagued with problems such as the parasitic
hydrogen evolution reaction, surface passivation, corrosion, and a rough metal electrode morphology
that is prone to short circuits. One strategy to overcome these issues is understanding surface processes
to facilitate more homogeneous electrodeposition of zinc by guiding the alignment of electrodeposited
zinc. Using Scanning Electrochemical Microscopy (SECM), the charge transport rate on zinc metal
anodes was mapped, demonstrating that manipulating electrolyte concentration can influence zinc
electrodeposition and solid electrolyte interphase (SEI) formation in ZMBs. Using XPS and Raman
spectroscopy, it is demonstrated that an SEI is formed on zinc electrodes at neutral pH, composed
primarily of a Zn4(OH)6SO4xH2O species, its formation being attributed to local pH increases at the
interface. This work shows that more extended high-rate cycling can be achieved using a 1 M ZnSO4
electrolyte and that these systems have a reduced tendency for soft shorts. The improved cyclability in
1 M ZnSO4 was attributed to a more homogeneous and conductive interface formed, rather than the
bulk electrolyte properties. This experimental methodology for studying metal battery electrodes is
transferable to lithium metal and anode-free batteries, and other sustainable battery chemistries such as
sodium, magnesium, and calcium.
