Chirality ("handedness") is a hot topic in surface science. In a new publication, we review the current state of knowledge about chirality in amino acid overlayers on copper surfaces, and present the findings of our own recent investigation of alanine on Cu{311} and {531}.
Read the press release here.
At a surface that has mirror symmetry, overall chirality can be imposed by adding a chiral adsorbate, such as an amino acid. Our work on symmetric Cu{311} exploits the arrangement of copper atoms in this surface to remove the complication of "footprint chirality", seen in the bonding of amino acids to Cu{110} or {100}. Under the right conditions, we found that alanine on Cu{311} self-organizes into an ordered structure, in which the packing of the molecules is governed by the symmetric footprint, rather than the chirality of the molecule. Small changes to the conditions, however, give rise to a pattern of chiral boundaries, the handedness of the boundary switching with the molecular handedness. In this case, chiral interactions between adjacent molecules propagate into long-range chirality in the self-organization.
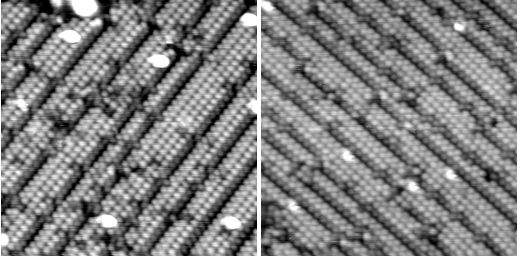 |
Overlayers of L-alanine (LH panel) and D-alanine (RH panel) on Cu{311}. |
One can also create metal surfaces that have no mirror symmetry: Cu{531} is such an intrinsically chiral surface. In principle, left- and right-handed molecules might interact differently with, say, a left-handed surface. This is exactly what we found when we deposited L- and D-alanine on Cu{531}-S. The bonding of the molecules to the surface, combined with hydrogen bonding between neighbouring molecules, is strong enough to cause restructuring of the arrangement of copper atoms. Crucially, the chiral phases that form are different depending on whether L- or D-alanine is used: a strong enantiospecific structural effect.
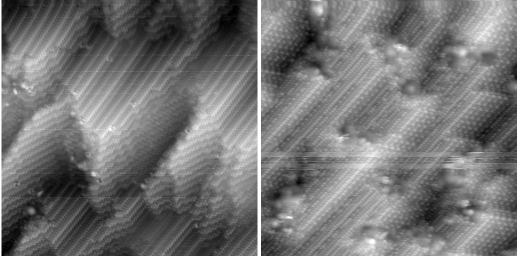 |
Overlayers of L-alanine (LH panel), D-alanine (RH panel) on Cu{531}. |
This is fundamental research, aimed at furthering our understanding of surface chirality at the level of individual atoms, molecules and bonds. Our work provides clear evidence for enantiospecific structural effects in molecule-surface interactions, effects that point towards a basis for new methods in chiral chemistry. In the longer term, we believe that these effects may be exploited practically, both for pharmaceutical synthesis using heterogeneous catalytic routes, and for enantio-sensitive bio-sensors.
We are delighted by the award of a new grant to continue this research, made by NSF and EPSRC to ourselves and to Jane Hinch (Rutgers University).
More about amino acids on Cu{311}
Following up our earlier work about the forms of chirality seen in amino acid overlayers on Cu surfaces, we have been looking in more depth at alanine - both enantiopure and racemic - and glycine on Cu{311}.
In our latest work, we have mapped out the conditions under which various L-alaninate structural phases are formed, using LEED and STM . We have also established the bonding configurations associated with these phases, using RAIRS measurements and DFT-based calculations of normal mode frequencies.
The most stable overlayer has the (2,1;1,2) structure that we reported before. Alaninate is bonded through the amine N and both carboxylate O atoms ('mu-3' bonding), accounting for all surface-layer Cu atoms at 0.33 ML coverage. At higher coverages, we now know that two ordered structures form, usually coexisting. These have (6,4;1,2) and (7,4;1,2) periodicities for L-alaninate; a fraction of the alaninate moieties are bonded through the amine N and just one of the carboxylate O atoms ('mu-2' bonding).
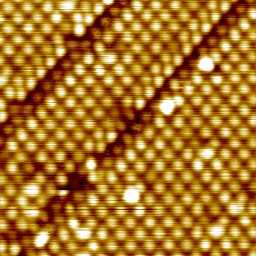 |
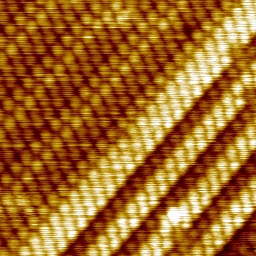 |
L-alaninate overlayers on Cu{311}. (2,1;1;2) structure, showing occasional translational domain boundaries (LH panel); coexisting (7,4;1,2) and (6,4;1,2) structures, (RH panel, upper left and lower right, respectively). (Images 10 nm x 10 nm, 78 K.) |
The (2,1;1,2) structure has a symmetric lattice, while the crystallography of the {311} surface orientation obviates the 'footprint chirality' seen for mu-3-bonded amino acids on Cu{110} and {100} surfaces. The higher-coverage structures, meanwhile, both have chiral lattices (i.e. breaking the clean-surface mirror symmetry), implying that chiral nearest-neighbour interactions lead to long-range chirality in the self-organisation.
The (2,1;1,2) structure at 0.33 ML is also seen when we move from enantiopure alanine to racemic alanine or to glycine. At higher coverages, periodic chiral-lattice structures are not seen, but chiral translational domain boundaries are seen in both cases. Racemic alaninate overlayers exhibit regions of L-like domains and regions of xD-like domains, alternating over a 10 nm length scale. The chiral boundaries seen with glycinate are even shorter (1-2 nm) and arranged less regularly, but occur despite the fact that glycine does not have a chiral centre. The primary effect of the presence/absence and position of the methyl group, therefore, is on the lengths of the boundaries.
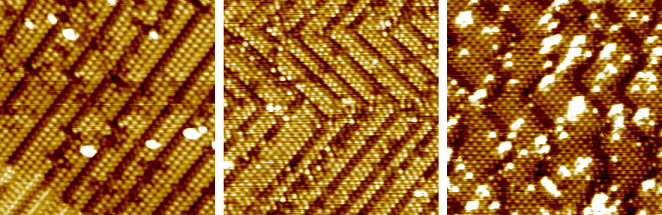 |
L-alaninate (LH), racemic alaninate (centre), glycinate (RH) overlayers on Cu{311}. (Each 20 nm x 20 nm, 78 K.) |
Atomic-scale roughness of an intrinsically chiral metal surface
We have used STM to reveal the atomically rough nature of an intrinsically chiral metal surface, Cu{531}. This is an example of a kinked-stepped fcc surface that is at an orientation devoid of all bulk mirror symmetry. One might therefore anticipate such surfaces having potential for enantiospecific heterogeneous catalysis involving chiral and pro-chiral molecular adsorbates.
Experimentally, we find that the clean Cu{531} departs strongly from ideal bulk termination. STM images recorded at 77 K show a high degree of atomic-scale roughness. Intact {531} meshes co-exist with a rich variety of steps, adatoms, vacancies, clusters and pits. Perhaps surprisingly, sharp LEED patterns can be recorded from such surfaces.
Simple nearest-neighbour bond-counting arguments rationalise the roughness. Creating a vacancy breaks six n-n bonds, while placing an adatom in a lattice site makes six n-n bonds. Adatom-vacancy annihilation therefore does not give the enthalpy reduction that normally drives ordering on low-index surfaces.
The thermal roughness of Cu{531} differs in detail from that of chiral kinked-stepped fcc surfaces having wider close-packed terraces, specifically the Cu and Pt{643} surfaces studied by other groups. We attribute the differences to repulsive step-step interactions: these oppose step meandering for small step-step spacings.
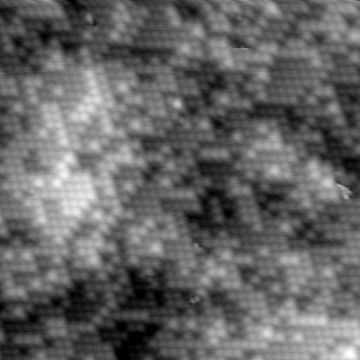 |
Figure 1. STM image of atomically rough Cu{531} at 77 K. Each bump is a Cu atom in the (local) outermost layer. Image size: 20 x 20 nm2. |
