Tissue calcification can be both physiological, e.g. bone, or pathological, e.g. vascular calcification (hardening of the arteries). Calcification in both contexts is the nucleation and growth of calcium phosphate deposits within the extracellular matrix, stiffening the tissue. 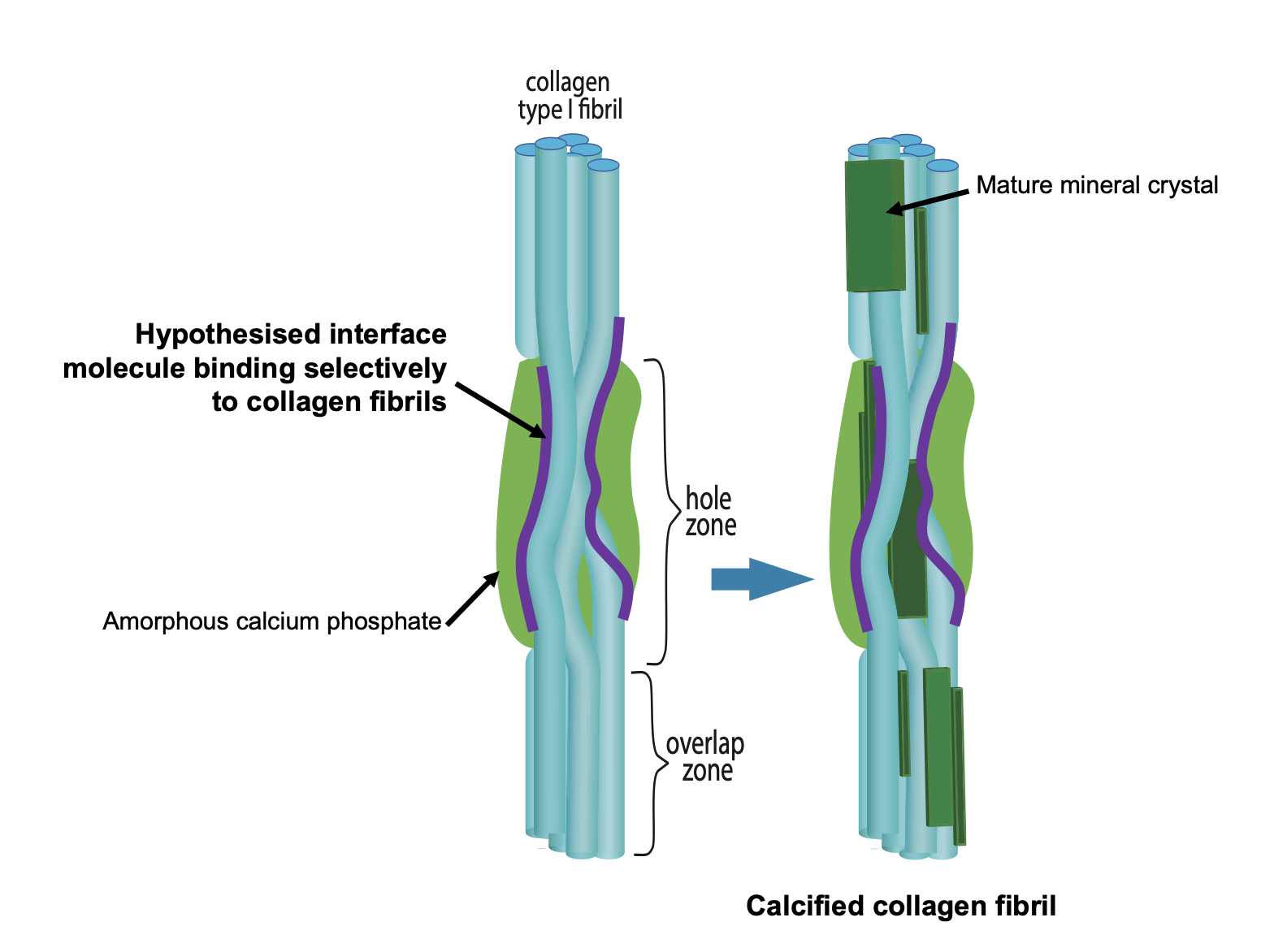
In physiological bone, the calcium phosphate mineral occurs as highly organized nanoscopic curved platelet-like structures arranged in layers around collagen fibrils, with further sub-structure within the platelets themselves. In pathological calcification, the extracellular matrix distribution and shape of the mineral structures is highly heterogeneous. In vascular calcification, for instance, calcification occurs in areas of vessel damage and can be organized around collagen fibrils – where they have not been degraded in the vessel damage – or in close association to the surfaces of elastin lamellae or as more isolated spherical structures.
Increasing evidence suggests that the delivery of calcium phosphate into the extracellular matrix in both physiological and pathological contexts comes from Ca-containing matrix vesicles and that the inorganic phosphate in the forming mineral deposit comes from enzymatic degradation of the vesicle phospholipid membrane. The ECM provides many possible “solid” nucleation surfaces for mineral formation. The complex chemical milieu in which mineral forms in the ECM provides (a) many organic ions, e.g. metabolic acid anions, that can potentially contribute to the mineral atomic structure, either by being physically incorporated into the mineral lattice or by binding to calcium ions and so perturbing the chemical equilibrium and (b) many soluble proteins and glycans that can bind to forming crystals, stabilize mineral crystal nuclei, further complicating the biomineralization process. Thus, ECM calcification in vivo cannot be understood in terms of crystal growth kinetics from simple calcium phosphate solutions nor can the mineral structures and compositions be properly understood in terms of purely inorganic calcium phosphate phases.
Figure 1. Schematic figure outlining the hypothesis that bone mineral nucleation occurs via a Ca-laden macromolecule binding selectively to collagen fibrils and thus locally concentrating calcium and phosphate ions in the vicinity of the PAR-collagen fibril binding sites.
How does mineral nucleate in the extracellular matrix?
A key question in bone calcification is how are collagen fibrils selected to be the substrate around which mineral forms out of all the possible nucleation sites in the extracellular matrix? We hypothesized that collagen is selected via a calcium-carrying macromolecule binding specifically to collagen fibrils (not collagen molecules) in bone calcification. Our heavy mouse model gave us the first clues as to what this biomacromolecule might be. We discovered that poly(ADP ribose) is synthesized by cells in both 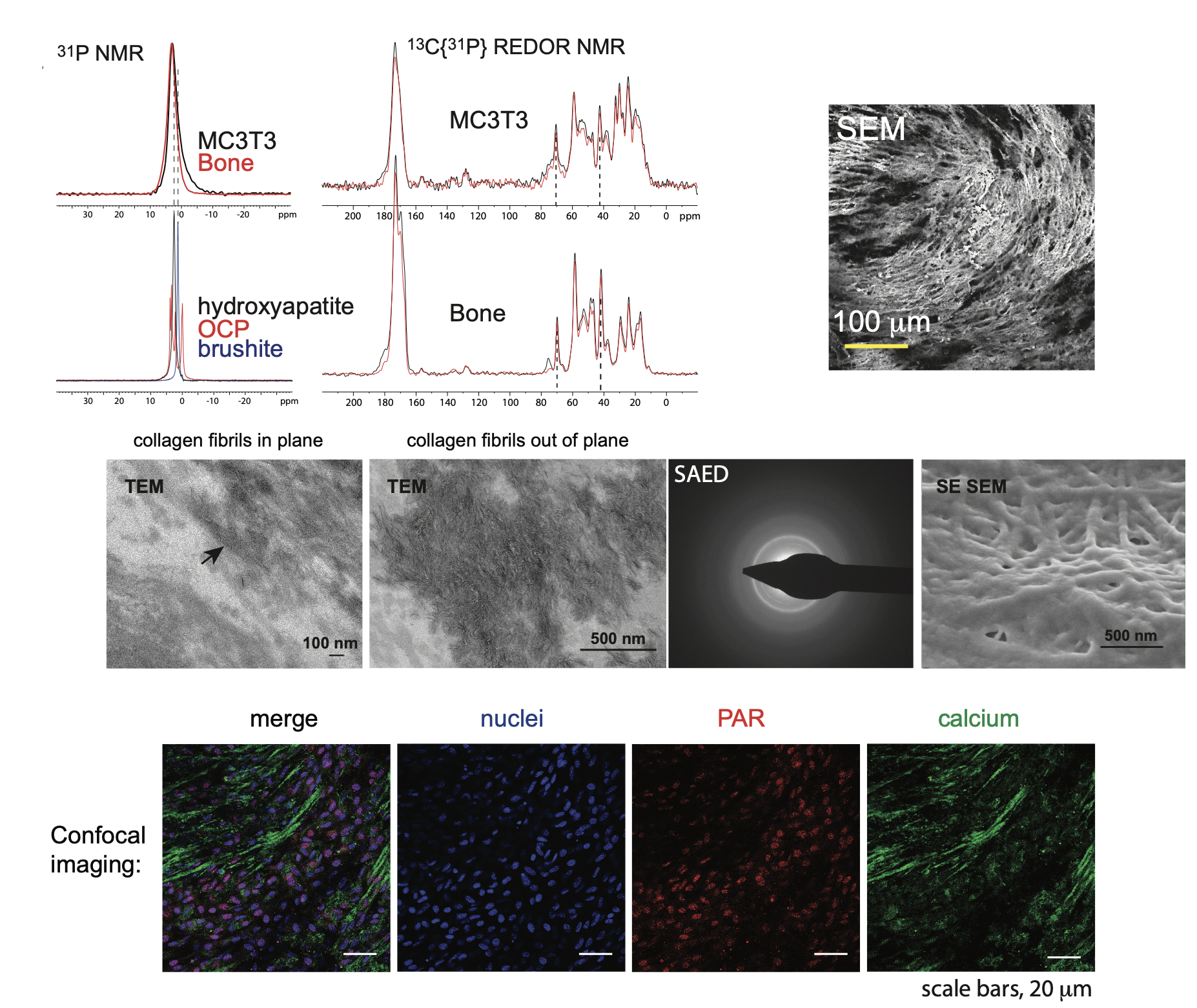 bone (osteoblasts) and vascular (vascular smooth muscle cells) calcification. In both these tissues, poly(ADP ribose) is detectable in the extracellular matrix during calcification, co-localizing with calcium phosphate and collagen fibrils in osteoblast cell cultures and spherical calcifying structures in vascular smooth muscle cell cultures. We are currently working on how poly(ADP ribose) is delivered to the extracellular matrix – in vascular smooth muscle cell matrix in vitro, it appears to be via matrix vesicles – and how it binds to collagen fibrils. A further area of work is how collagen glycation affects poly(ADP ribose) binding to collagen fibrils and subsequent calcification to understand the deficiencies in bone mineralization in ageing and diabetes.
bone (osteoblasts) and vascular (vascular smooth muscle cells) calcification. In both these tissues, poly(ADP ribose) is detectable in the extracellular matrix during calcification, co-localizing with calcium phosphate and collagen fibrils in osteoblast cell cultures and spherical calcifying structures in vascular smooth muscle cell cultures. We are currently working on how poly(ADP ribose) is delivered to the extracellular matrix – in vascular smooth muscle cell matrix in vitro, it appears to be via matrix vesicles – and how it binds to collagen fibrils. A further area of work is how collagen glycation affects poly(ADP ribose) binding to collagen fibrils and subsequent calcification to understand the deficiencies in bone mineralization in ageing and diabetes.
Figure 2. We developed an in vitro model of osteoblast matrix calcification (MC3T3 E1 cells) to probe the mechanism of matrix calcification. Our interdisciplinary approach integrates many different characterization techniques, such as solid state NMR spectroscopy (top left), electron microscopy (Scanning electron microscopy, SEM, top right; Transmission electron microscopy, middle), and immunofluoresence (bottom)
Selected publications:
- Poly(ADP-Ribose) Links the DNA Damage Response and Biomineralization. K Mueller, R Hayward, R Rajan, M Whitehead, S Ahmad, A Cobb, M Sun, I Goldberga, R Li, U Bashtanova, A M. Puszkarska, D G. Reid, R A. Brooks, J N. Skepper, J Bordoloi, W Ying Chow, H Oschkinat, A Groombridge, O A. Scherman, J A. Harrison, A Verhulst, P C. D’Haese, E Neven, LM Needham, S F. Lee, C M. Shanahan*, M J. Duer – Cell Reports (2019) 27, 3124 (DOI: 10.1016/j.celrep.2019.05.038)
- NMR spectroscopy of native and in vitro tissues implicates polyADP ribose in biomineralization. WY Chow, R Rajan, KH Muller, DG Reid, JN Skepper, WC Wong, RA Brooks, M Green, D Bihan, RW Farndale, DA Slatter, CM Shanahan, MJ Duer – Science (2014) 344, 742 (DOI: 10.1126/science.1248167)
The structure of bone mineral
The complex chemical milieu in which bone mineral forms means that there are many coupled chemical reactions contributing to its formation. Furthermore, the mineral formation takes place in an environment where there is likely restricted diffusion, and so chemical equilibria are unlikely to be reached. These two factors make bone mineral highly dynamic in terms of its composition and structure - consistent with the daily turnover of bone mineral and bones acting as the body's buffer for calcium homeostasis.
Bone mineral has been known since the 1960s to contain metabolic anions associated with cellular respiration: carbonate (from carbon dioxide), citrate and lactate are the most abundant such species in bone mineral.
In 2014, we proposed a new model for bone mineral based on octacalcium phosphate-citrate, and then added further complexity to this model with mineral phases formed by transformation of octacalcium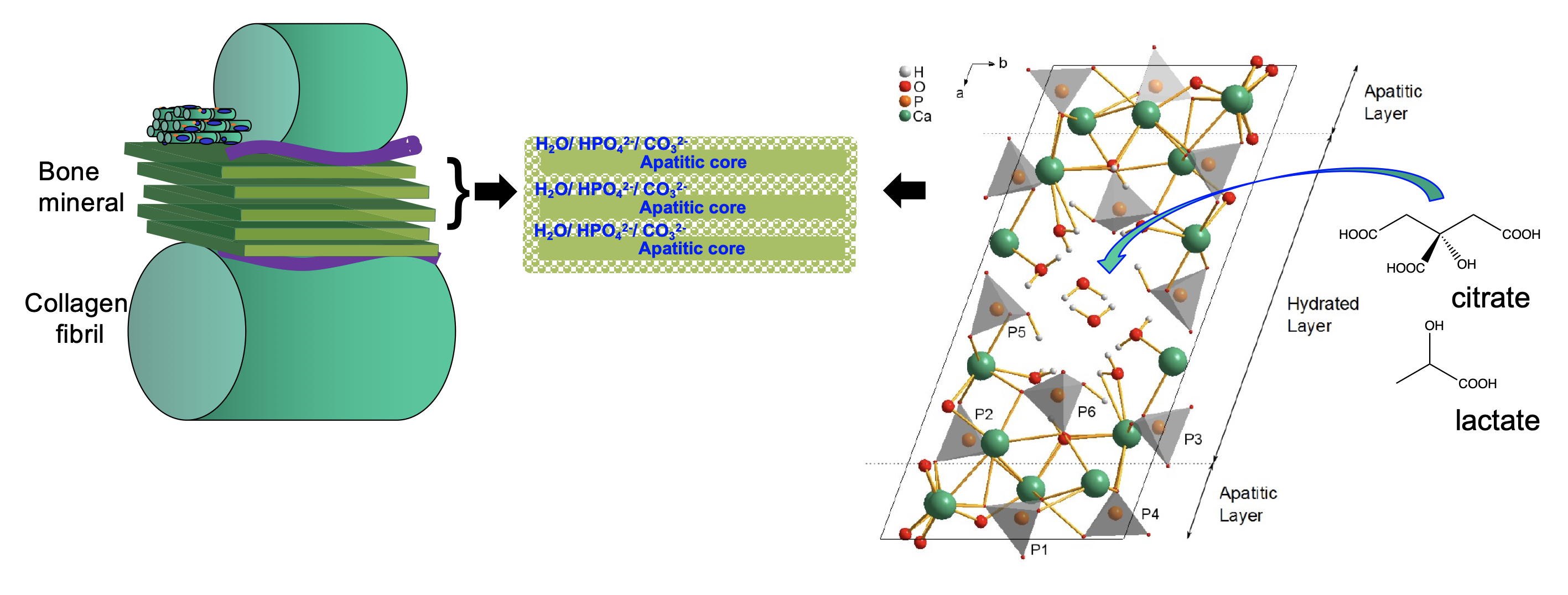 phosphate towards hydroxyapatite in the presence of citrate, lactate and carbonate. The incorporation of citrate and lactate control the hardness and solubility of the mineral, citrate increasing the hardness and lactate significantly decreasing it. This is consistent with highly dense bone mineral correlating with high blood plasma citrate concentrations in humans and high relative lactate concentration in (soft) foetal bone. Intriguingly, some species, notable freshwater turtles, sequester carbon dioxide and lactate into their skeletons when in hypoxic conditions, i.e. underwater for long periods of time. Skeletal bone acts as the body’s calcium pool to ensure calcium homeostasis, and so calcium ions must dissolve from bone mineral during periods of high calcium demand in the body and re-deposit during periods of low calcium demand. Such processes take place on a daily basis and so there must be exquisite control over bone mineral solubility - and we surmise that the coupling of the bone mineral solubility to cellular respiration/ energy demands are part of this control system.
phosphate towards hydroxyapatite in the presence of citrate, lactate and carbonate. The incorporation of citrate and lactate control the hardness and solubility of the mineral, citrate increasing the hardness and lactate significantly decreasing it. This is consistent with highly dense bone mineral correlating with high blood plasma citrate concentrations in humans and high relative lactate concentration in (soft) foetal bone. Intriguingly, some species, notable freshwater turtles, sequester carbon dioxide and lactate into their skeletons when in hypoxic conditions, i.e. underwater for long periods of time. Skeletal bone acts as the body’s calcium pool to ensure calcium homeostasis, and so calcium ions must dissolve from bone mineral during periods of high calcium demand in the body and re-deposit during periods of low calcium demand. Such processes take place on a daily basis and so there must be exquisite control over bone mineral solubility - and we surmise that the coupling of the bone mineral solubility to cellular respiration/ energy demands are part of this control system.
Current work in the group is exploring the roles of metabolic anions in bone mineral properties in more detail to understand how bone mineral structure and solubility are affected by ageing and cancer.
Figure 3. Schematic of new model for bone mineral structure based on octacalcium phosphate-citrate. The structure of octacalcium phosphate citrate using NMR crystallography and solid state NMR spectroscopy.
Selected publications:
- Citrate bridges between mineral platelets in bone E Davies, KH Müller, WC Wong, CJ Pickard, DG Reid, JN Skepper, MJ Duer – Proceedings of the National Academy of Sciences (2014)111, E1354 (DOI: 10.1073/pnas.1315080111)
- Citrate occurs widely in healthy and pathological apatitic biomineral: mineralized articular cartilage, and intimal atherosclerotic plaque and apatitic kidney stones. DG Reid, MJ Duer, GE Jackson, RC Murray, AL Rodgers, CM Shanahan – Calcified Tissue International (2013) 93, 1 (DOI: 10.1007/s00223-013-9751-5)
- Applications of NMR Crystallography to Problems in Biomineralization: Refinement of the Crystal Structure and 31P Solid-State NMR Spectral Assignment of Octacalcium Phosphate E Davies, MJ Duer, SE Ashbrook, JM Griffin – Journal of the American Chemical Society (2012) 134, 12508 (DOI: 10.1021/ja3017544)
- Solid state NMR - An indispensable tool in organic-inorganic biocomposite characterization; refining the structure of octacalcium phosphate composites with the linear metabolic di-acids succinate and adipate. Y Li, DG Reid, MJ Duer, JCC Chan – Solid State Nucl Magn Reson (2018) 95, 1 (DOI: 10.1016/j.ssnmr.2018.08.004)
- The contribution of solid-state NMR spectroscopy to understanding biomineralization: Atomic and molecular structure of bone MJ Duer – Journal of Magnetic Resonance (2015) 253, 98 (DOI: 10.1016/j.jmr.2014.12.011)
Vascular calcification
We have collaborated with internationally-leading vascular biologist, Professor Catherine Shanahan (https://www.kcl.ac.uk/people/catherine-shanahan) since 2003 to understand the molecular mechanisms involved in vascular calcification (hardening of the arteries). This widespread human condition causes elevated blood pressure, is an independent risk factor for cardiovascular disease and can be causal in stroke and thrombosis. Its prevalence increases with age and is accelerated in diabetes and chronic kidney disease. It can be associated with damage to the lining of an artery from atherosclerosis (intimal or atherosclerotic calcification) or oxidative stress in vascular smooth muscle cells in the medial arterial layer (ageing, diabetes and chronic kidney disease).
Chondro-osteogenic differentiation of vascular smooth muscle cells is a pre-requisite to vascular 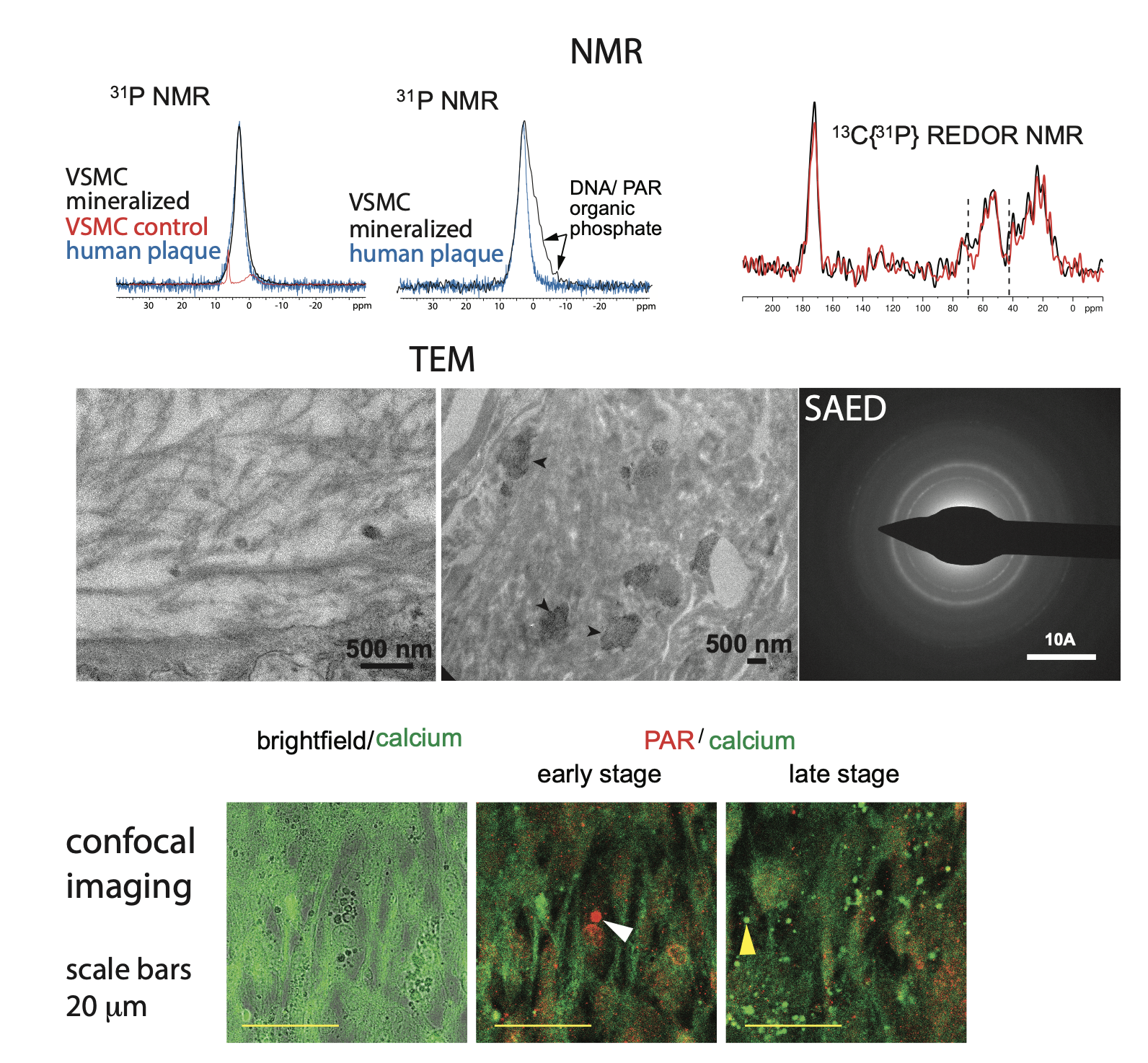 calcification. Our current working model of vascular calcification is oxidative stress in vascular smooth muscle cells in both atherosclerotic and medial vascular calcification leading to DNA damage, which signals the production of poly(ADP ribose) by PARPs 1 and 2, particularly the latter. PARylation of nuclear proteins leads to osteogenic differentiation, which includes Ca-containing matrix vesicles being released into the extracellular matrix. Extracellular poly(ADP ribose) appears to be in spherical vesicle-like structures, suggesting that the matrix vesicles may be the sources of extracellular poly(ADP ribose) as well as calcium ions. Our working hypothesis is that Ca-laden poly(ADP ribose) droplets from matrix vesicles lodge in the vascular extracellular matrix and solidify/ crystallize into mineral particles over time in the vasculature (6).
calcification. Our current working model of vascular calcification is oxidative stress in vascular smooth muscle cells in both atherosclerotic and medial vascular calcification leading to DNA damage, which signals the production of poly(ADP ribose) by PARPs 1 and 2, particularly the latter. PARylation of nuclear proteins leads to osteogenic differentiation, which includes Ca-containing matrix vesicles being released into the extracellular matrix. Extracellular poly(ADP ribose) appears to be in spherical vesicle-like structures, suggesting that the matrix vesicles may be the sources of extracellular poly(ADP ribose) as well as calcium ions. Our working hypothesis is that Ca-laden poly(ADP ribose) droplets from matrix vesicles lodge in the vascular extracellular matrix and solidify/ crystallize into mineral particles over time in the vasculature (6).
Figure 3 (top) Solid-state NMR data from our in vitro vascular smooth muscle cell in vitro model of vascular calcification. (middle) TEM images show both calcified collagen fibrils and “patches” of mineral (arrow heads) in regions of disorganized/ degraded collagen in the same model. (bottom) Confocal imaging of the calcification in our in vitro model.
The key involvement of PARP2 in the vascular calcification process led us to propose PARP2 inhibition as a therapeutic strategy to treat vascular calcification. We showed that minocycline, a selective PARP2 inhibitor, inhibits vascular smooth muscle cell-driven calcification in vitro and in vivo. As a result of this work, a patient trial with minocycline is in progress at Addenbrookes Hospital in Cambridge.
Selected publications:
- Poly(ADP-Ribose) Links the DNA Damage Response and Biomineralization. K Mueller, R Hayward, R Rajan, M Whitehead, S Ahmad, A Cobb, M Sun, I Goldberga, R Li, U Bashtanova, A M. Puszkarska, D G. Reid, R A. Brooks, J N. Skepper, J Bordoloi, W Ying Chow, H Oschkinat, A Groombridge, O A. Scherman, J A. Harrison, A Verhulst, P C. D’Haese, E Neven, LM Needham, S F. Lee, C M. Shanahan*, M J. Duer – Cell Reports (2019) 27, 3124 (DOI: 10.1016/j.celrep.2019.05.038)
- The effect of particle agglomeration on the formation of a surface-connected compartment induced by hydroxyapatite nanoparticles inhuman monocyte-derived macrophages KH Müller, M Motskin, AJ Philpott, AF Routh, CM Shanahan, MJ Duer, JN Skepper – Biomaterials (2014) 35, 1074 (DOI: 10.1016/j.biomaterials.2013.10.041)
- Citrate occurs widely in healthy and pathological apatitic biomineral: mineralized articular cartilage, and intimal atherosclerotic plaque and apatitic kidney stones. DG Reid, MJ Duer, GE Jackson, RC Murray, AL Rodgers, CM Shanahan – Calcified Tissue International (2013) 93, 1 (DOI: 10.1007/s00223-013-9751-5)
- Mineral surface in calcified plaque is like that of bone: further evidence for regulated mineralization. MJ Duer, T Friscić, D Proudfoot, DG Reid, M Schoppet, CM Shanahan, JN Skepper, ER Wise – Arteriosclerosis, Thrombosis, and Vascular Biology (2008) 28, 2030 (DOI: 10.1161/atvbaha.108.172387)
- DNA Damage Response M Duer, AM Cobb, CM Shanahan – Arteriosclerosis Thrombosis and Vascular Biology (2020) 40, e193 (DOI: 10.1161/atvbaha.120.313792)
