The oxidation of surfaces is a fascinating topic, whether one's focus rests upon corrosion, catalytic activity or the resulting oxide's physical properties. An important example of the latter motivation is to be found in the oxidation of silicon, which is a key step in the fabrication of integrated circuits for electronic applications. Oxidation by exposure to "ordinary" oxygen (O2, or "dioxygen") is the standard industrial approach, but recent experimental work has suggested that exposure to ozone (O3) would produce higher-quality oxide films. It is well-known that ozone is generally much more reactive than dioxygen, but the details of its attack on the silicon surface were unknown prior to our molecular dynamics simulations.
To simulate the interaction of ozone with silicon surfaces, we used first-principles density functional theory to calculate the forces acting on each atom in the system. The phrase "first-principles" means that these forces were derived from a quantum mechanical foundation, with no adjustable parameters being tweaked to "improve" the results. Using the calculated forces, we were then able to calculate the acceleration of the atoms, and hence to follow the trajectories they follow over a period of time. In view of the computational expense of first-principles molecular dynamics, our trajectories typically only covered one or two picoseconds of motion (one picosecond is one millionth of a millionth of one second) but this is enough to capture the essence of ozone's very rapid interaction with the surface.
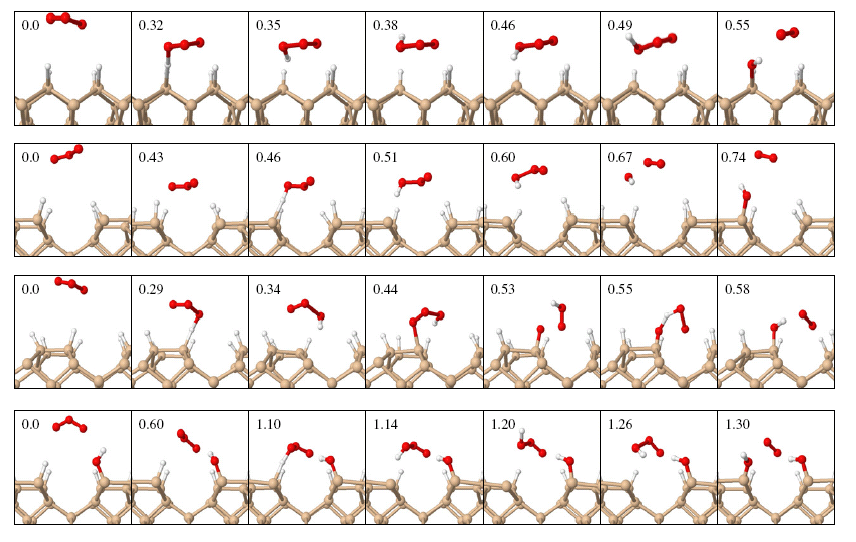
Our simulated trajectories show that ozone reacts easily with the clean silicon surface, dissociating either fully (to leave three separate oxygen atoms on the surface) or partially (to leave a single oxygen atom on the surface, with a dioxygen molecule left to float away). Even more intriguing, however, is the behaviour of ozone on a silicon surface that has been treated with hydrogen. Deposition of hydrogen on silicon surfaces makes them much less prone to oxidation by ordinary dioxygen, but ozone succeeds in reacting despite the presence of hydrogen, albeit in a very unusual fashion. First, the ozone molecule "steals" a hydrogen atom from the surface, forming a highly unstable compound known as a "radical", and leaving a reactive hydrogen-free site on the surface. The HO3 radical normally has a lifetime of microseconds (millionths of a second) but the reactive surface site here induces it to dissociate within a few tenths of a picosecond. The result is that the reactive surface site forms a bond with an -OH group, while once again dioxygen floats away unreacted.
Snapshots from four trajectories showing the reaction of ozone with hydrogen-passivated silicon. The numbers indicate elapsed time in picoseconds.
Further work has focussed upon the oxidation of diamond surfaces, where the strong bonds between carbon atoms lead to a much lower reactivity than was the case for silicon. Here, ozone has potential in attempts to "dope" the surface, meaning to change its electronic properties by adding small numbers of impurity atoms (oxygen atoms, in this case).
