Industrial ammonia synthesis by the Haber-Bosch process is energy-intensive, requiring high temperatures and pressures. In the nitrogen cycle, by contrast, nitrogen fixation occurs under ambient conditions. Whereas the industrial catalyst is iron-based, the reactive centre (the 'FeMo-cofactor') in nitrogenase - the enzyme that catalyses the conversion of N2 into NH3 - is in essence an iron-sulphur nanocluster.
A bio-inspired approach to ammonia synthesis therefore suggests iron sulphide surfaces as good potential candidates for the catalyst. As a starting point for investigating this intriguing possibility, we chose to examine N-based chemistry on the {100} surfaces of iron pyrite, FeS2. Whilst an FeS2{100} surface is clearly only loosely related to the FeMo-cofactor, iron pyrite is cheap and abundant. The habit plane of naturally-occurring FeS2 crystals is {100}, and it is possible (with some care) to prepare well-defined FeS2{100} surfaces in UHV.
 |
Ball model of FeS2{100} surface. |
Our experiments showed that FeS2{100} surfaces will not dissociate either N2, even at temperatures up to 450 K and pressures up to 1 bar, or H2. At first sight, this would seem to indicate that FeS2{100} surfaces are not in any way promising for NH3 synthesis.
However, it is possible to get atomic N and H onto the surface by other means. To obtain Nads, we passed N2 through an ion gun to obtain 'activated nitrogen'. This leads to considerable crystallographic disorder, and two nitrogen species which we attribute to subsurface atomic N in both interstitial and substitutional sites. Hads could be obtained by cracking H2 at a hot filament.
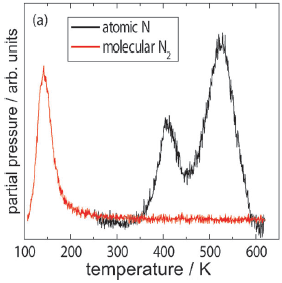 |
TPRS data showing N2 desorption from FeS2{100}. Molecularly adsorbed N2 desorbs at 130 K. Recombinative desorption of Nads gives peaks at 410 K and at 520 K. |
The crucial experiment was to implant N in this way, and then adsorb H, with the surface below 200 K. A subsequent TPR spectrum revealed (i) that NH3 was evolved, and (ii) that this occurred below room temperature.
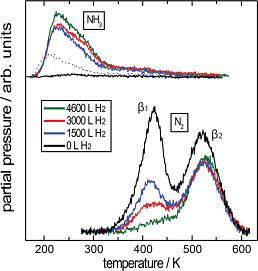 |
TPRS data recorded after exposing FeS2{100}, at 200 K, first to activated nitrogen and then to atomic H. NH3, produced by surface reaction of adsorbed atomic N and H, desorbs between 200 and 300 K. |
The Haber-Bosch process has been studied intensively over many years. Seminal work by Emmett and by Taylor in the 1930s identified N2 dissociation as the rate-determining step, a view reinforced by the work of Ertl and co-workers in the 1970s and 80s; Nørskov and co-workers showed that the low sticking probability for nitrogen on Fe surfaces is due to a high entropic barrier to dissociation. Strongin and Somorjai argued that difficulties in desorbing NH3 are also a significant problem. More recently, a team led by King, Jenkins and Wales has focussed on the barriers to sequential hydrogenation of Nads.
The significance of our measurements on FeS2{100} is that the hydrogenation steps and the desorption of NH3 take place below room temperature, indicating that these processes are more facile than on Fe surfaces. In consequence, whilst iron pyrite is not a complete ammonia synthesis catalyst, it does show real promise for several of the key mechanistic steps. It may therefore have an important role to play in a new, low-energy route to ammonia synthesis.
