 Physiosorption of large molecules onto surfaces has been an area of longstanding interest for the surface science group. Simple Van der Walls forces act to keep the molecules attached to the surface, while a variety of intermolecular interactions can lead to spontaneous self-assembly in the plane parallel to the surface. Confinement to two dimensions leads to novel phase behaviour, as well as allowing intermolecular interactions to be more easily characterised.
Physiosorption of large molecules onto surfaces has been an area of longstanding interest for the surface science group. Simple Van der Walls forces act to keep the molecules attached to the surface, while a variety of intermolecular interactions can lead to spontaneous self-assembly in the plane parallel to the surface. Confinement to two dimensions leads to novel phase behaviour, as well as allowing intermolecular interactions to be more easily characterised.
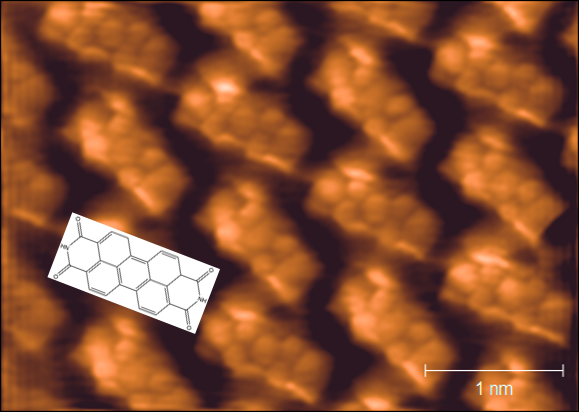
Using an Omicron LT-STM it is possible to image such monolayers down to atomic resolution, greatly aiding interpretation of structure in the surface plane. An image of the hydrogen bonding molecule PTCDI shows the power of this technique.
More recent work has focused on halogen bonds, the comparatively understudied cousin of the hydrogen bond. Halogen bonding is a function of the anisotropic charge distribution around a halogen atom (X) bonded to a substituent (Y). If Y is sufficiently electron withdrawing, a σ-hole of partial positive charge develops on X, on the opposite face to that facing Y. This can be considered as being due to the relatively low energy of the σ* antibonding molecular orbital (MO).
Such a σ-hole can then accept electrons from a lone pair, leading to a favourable halogen bonding interaction. These interactions are of the same order of magnitude as hydrogen bonds, with greater than 20 kJ mol-1 per bond being typical for the systems studied. The details of the charge distribution around the halogen also mean that such bonds are much more directional than their hydrogen bond equivalents, a key advantage for crystal engineering.
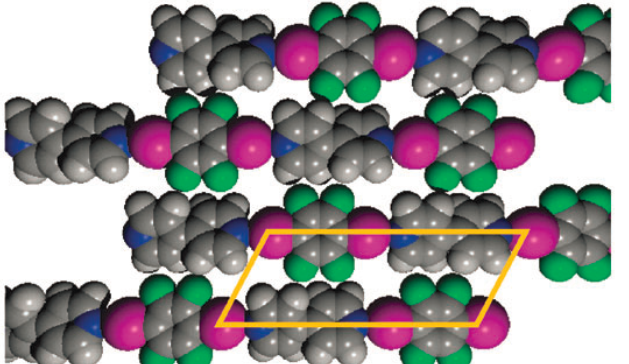
As part of a collaboration with the Clarke group, DFT simulations were used to help quantify the strength of interactions in simple monolayers. The long infinite chains formed between 1,4-diiodotetrafluorobenzene (DITFB) and 4,4’- bipyridine (BPY) are pictured.
In the long term it is hoped the application of STM and DFT will aid in design of novel molecular architectures. In particular it is hoped to form porous networks. Such structures must overcome the general close packing principle, but have been observed in hydrogen bonded species such as between PTCDI and melamine. The comparable strength and greater directionality of the halogen bond should aid design of analogous structures.
(1) Sacchi, M.; Brewer, A. Y.; Jenkins, S. J.; Parker, J. E.; Friščić, T.; Clarke, S. M. Langmuir, 2013, 29 (48), pp 14903–14911
(2) Brewer, A. Y.; Sacchi, M.; Parker, J. E.; Truscott, C. L.; Jenkins, S. J.; Clarke, S. M. Phys. Chem. Chem. Phys. 2014, 16, 19608–19617.
