Battery Applications
Introduction
We are investigating a variety of electrode materials for applications in lithium-ion, sodium-ion and magnesium-ion batteries. For lithium-ion based systems, materials under investigation include intercalation compounds such as LiFePO4, Li-Ni-Co-Al-O (NCA), LiMnO2, TiO2-B and Nb2O5; conversion materials such as VS4, NbS3, FeF3 and RuO2; and alloying materials such as P, Ge and Si. Additionally, we study potential alternatives to the conventional lithium-ion battery systems including metal-air, sodium-ion and divalent magnesium-ion batteries. In this "beyond lithium" research, we study, among others, lithium-air, sodium-air, NaMnO2, Na-Fe-SO4, Na-hard carbons, Na-P, Na-Sn, Na-Sb, Mg-Bi, Mg6MnO8 and Mg electrolytes. We utilize an array of advanced characterisation tools and theoretical techniques to understand complex reaction mechanisms in these battery systems and to develop new materials.
List of selected publications:
"Materials' Methods: NMR in Battery Research", O Pecher, J Carretero-Gonzalez, KJ Griffith and CP Grey, Chem. Mater. (2017) 29, 213 (DOI: 10.1021/acs.chemmater.6b03183)
Lithium Ion Batteries (LIBs)
Rechargeable lithium-ion batteries recently celebrated their 25th anniversary as commercial products. However, mobile technologies, electric vehicles, safety, cost, sustainability and large-scale storage all provide the impetus for research into new battery materials and mechanisms. We are interested in a wide variety of commercial and next-generation cathode and anode materials, solid electrolytes for all-solid state batteries, and Li dendrite growth mechanisms.
In our group, laboratory-based and synchrotron/neutron diffraction methods are used extensively to understand the changes in average 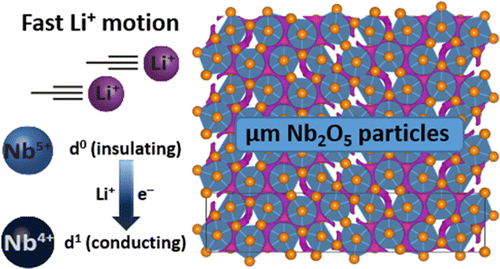 structure that occur during electrochemical discharge/charge reactions. Lithium has two NMR active isotopes, 6Li and 7Li, that provide direct insight into the local environment and dynamics of lithium in topotactic or conversion/alloying electrodes. The application of two-dimensional exchange spectroscopy or variable-temperature relaxation measurements enables quantitative analysis of lithium ion motion and often yields information on diffusion pathways and mechanisms. In alloying and conversion electrodes, the amorphous nature of starting materials and intermediates often hinders diffraction studies but NMR and Pair Distribution Function (PDF) analysis are sensitive to local structure and can be used for phase identification. Ab initio calculations of phase space and/or NMR parameters frequently guide and support experimental studies. Indeed, in these complex systems, several complementary techniques must often be applied to provide a detailed understanding of the changes that occur and an accurate description of the phases present in a lithium-ion battery during lithiation and delithiation cycles.
structure that occur during electrochemical discharge/charge reactions. Lithium has two NMR active isotopes, 6Li and 7Li, that provide direct insight into the local environment and dynamics of lithium in topotactic or conversion/alloying electrodes. The application of two-dimensional exchange spectroscopy or variable-temperature relaxation measurements enables quantitative analysis of lithium ion motion and often yields information on diffusion pathways and mechanisms. In alloying and conversion electrodes, the amorphous nature of starting materials and intermediates often hinders diffraction studies but NMR and Pair Distribution Function (PDF) analysis are sensitive to local structure and can be used for phase identification. Ab initio calculations of phase space and/or NMR parameters frequently guide and support experimental studies. Indeed, in these complex systems, several complementary techniques must often be applied to provide a detailed understanding of the changes that occur and an accurate description of the phases present in a lithium-ion battery during lithiation and delithiation cycles.
List of selected publications:
"Evolution of Structure and Lithium Dynamics in LiNi0.8Mn0.1Co0.1O2 (NMC811) Cathodes during Electrochemical Cycling", K Märker, PJ Reeves, C Xu, KJ Griffith, CP Grey – Chemistry of Materials (2019) 31, 2545 (DOI: 10.1021/acs.chemmater.9b00140)
"7Li NMR Chemical Shift Imaging To Detect Microstructural Growth of Lithium in All-Solid-State Batteries", LE Marbella, S Zekoll, J Kasemchainan, SP Emge, PG Bruce, CP Grey – Chemistry of Materials (2019) 31, 2762 (DOI: 10.1021/acs.chemmater.8b04875)
"Unraveling the Reaction Mechanisms of SiO Anodes for Li-Ion Batteries by Combining in Situ Li-7 and ex Situ Li-7/Si-29 Solid-State NMR Spectroscopy", K Kitada, O Pecher, PCMM Magusin, MF Groh, RS Weatherup, CP Grey – J Am Chem Soc (2019) 141, 7014 (DOI: 10.1021/jacs.9b01589)
"Understanding Fluoroethylene Carbonate and Vinylene Carbonate Based Electrolytes for Si Anodes in Lithium Ion Batteries with NMR Spectroscopy", Y Jin, N-JH Kneusels, LE Marbella, E Castillo-Martínez, PCMM Magusin, RS Weatherup, E Jónsson, T Liu, S Paul, CP Grey – Journal of the American Chemical Society (2018) 140, 9854 (DOI: 10.1021/jacs.8b03408)
"Niobium Tungsten Oxides for High-rate Lithium-ion Charge Storage", KJ Griffith, KM Wiaderek, G Cibin, LE Marbella, CP Grey - Nature (2018) 559, 556 (DOI: 10.1038/s41586-018-0347-0)
"Sustainability and In Situ Monitoring in Battery Development", CP Grey and JM Tarascon, Nature Mater. (2017) 16, 45 (DOI: 10.1038/nmat4777)
"Preventing Structural Rearrangements on Battery Cycling: A First-Principles Investigation of the Effect of Dopants on the Migration Barriers in Layered Li0.5MnO2", ID Seymour, DJ Wales and CP Grey, J. Phys. Chem. C (2016) 120, 19521 (DOI: 10.1021/acs.jpcc.6b05307)
"High-rate Intercalation without Nanostructuring in Metastable Nb2O5 Bronze Phases", KJ Griffith, AC Forse, JM Griffin and CP Grey, J. Am. Chem. Soc. (2016) 138, 888 (DOI: 10.1021/jacs.6b04345)
"Unraveling the Complex Delithiation Mechanisms of Olivine-Type Cathode Materials, LiFexCo1-xPO4", FC Strobridge, H Liu, M Leskes, OJ Borkiewicz, KM Wiaderek, PJ Chupas, KW Chapman and CP Grey, Chem. Mater. (2016) 28, 2676 (DOI: 10.1021/acs.chemmater.6b00319)
Li-excess Cathodes for Li-Ion Batteries
Since 2001 and the synthesis of the family of Li‑excess materials (i.e. those with a Li:TM ratio >1) by the group of Dahn, a broad area in the search for the next generation of Li-ion cathodes has opened up1. It has been shown that by incorporating Li into the TM‑layer can give yield materials with extremely high specific capacity,2,3 for example the Li–Mn–Ni family of compounds with the general formula Li[Ni x Li(1/3-2x/3)Mn(2/3-x/3)]O2 have demonstrated reversible capacities of up to 230 mAhg-1, exceeding the theoretical capacity if only the transition metals are considered redox active4. There are however a number of draw backs that inhibit their implementation. Very high first charge capacity loss, poor rate capability and a fade in the average voltage, leading to a loss of power output, with each cycle.5
This exciting family of materials has been the focus of a lot of work in our group. By using techniques that are highly sensitive to the local structure we are studying the structural and electronic characteristics of these compounds that give rise to their unusual properties. Recent work includes understanding the effects and causes of local ordering in otherwise highly disordered compounds.6 As well as developing the use of 17O NMR for studying role the oxygen lattice plays in these compounds.7,8
Reference:
1. "Layered Cathode Materials Li[NixLi(1/3−2x/3)Mn(2/3−x/3)]O2 for Lithium-Ion Batteries", Lu, Z.; MacNeil, D. D.; Dahn, J. R., Electrochem. Solid-State Lett. 2001, 4 (11), A191, (DOI: 10.1149/1.1407994)
2. "Layered Lithium Insertion Material of LiNi1/2Mn1/2O2: A Possible Alternative to LiCoO2 for Advanced Lithium-Ion Batteries", Ohzuku, T.; Makimura, Y., Chem. Lett. 2001, 2, 744–745.(DOI: 10.1246/cl.2001.744)
3. "Synthesis, Structure, and Electrochemical Behavior of Li[NixLi[1/3−2x/3]Mn[2/3−x/3]]O2", Lu, Z.; Beaulieu, L. Y.; Donaberger, R. a.; Thomas, C. L.; Dahn, J. R., J. Electrochem. Soc. 2002, 149, A778. (DOI: 10.1016/j.jpowsour.2006.10.022)
4. "Countering the Voltage Decay in High Capacity XLi2MnO3●(1–x)LiMO2 Electrodes (M=Mn, Ni, Co) for Li+-Ion Batteries", Croy, J. R.; Kim, D.; Balasubramanian, M.; Gallagher, K.; Kang, S.-H.; Thackeray, M. M., J. Electrochem. Soc. 2012, 159 (6), A781 (DOI: 10.1149/2.080206jes)
List of selected publications:
5. "Short-Range Ordering in Battery Electrode, the ‘Cation-Disordered’ Rocksalt Li1.25Nb0.25 Mn0.5O2", Jones, M. A.; Reeves, P. J.; Seymour, I. D.; Cliffe, M.; Dutton, S. E. E.; Grey, C. P., Chem. Commun. 2019, 2–5. (DOI: 10.1039/C9CC04250D)
6. "Characterizing Oxygen Local Environments in Paramagnetic Battery Materials via 17O NMR and DFT Calculations", Seymour, I. D.; Middlemiss, D. S.; Halat, D. M.; Trease, N. M.; Pell, A. J.; Grey, C. P., J. Am. Chem. Soc. 2016, 138 (30), 9405–9408. (DOI: 10.1021/jacs.6b05747)
7. "Characterizing the Structure and Phase Transition of Li 2 RuO 3 Using Variable-Temperature 17 O and 7 Li NMR Spectroscopy", Reeves, P. J.; Seymour, I. D.; Griffith, K. J.; Grey, C. P., Chem. Mater. 2019, 31 (8), 2814–2821. (DOI: 10.1021/acs.chemmater.8b05178)
Sodium Ion Batteries (NIBs)
Lithium-ion batteries (LIBs) have dominated the marketplace in batteries for devices since the 1990s, as LIBs offer the highest known energy density and output voltages of all known rechargeable batteries. However, global lithium reserves are limited, and mostly located in politically unstable regions: relying on these countries to produce the world’s future energy storage systems should be avoided. In addition, Li extraction is an energy intensive process, which causes significant environmental damage, for example by draining and evaporating water from already arid regions of South America.
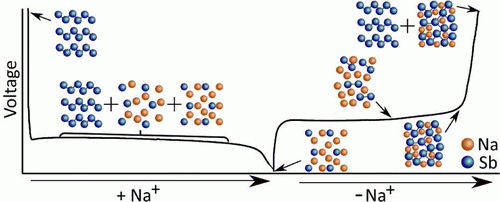 A cheaper and more sustainable alternative to LIBs are sodium-ion batteries (NIBs). Sodium does not suffer such limitations on global reserves, and its abundance means NIBs can be constructed at a lower cost than LIBs. The low production costs make NIBs ideally suited to grid-scale storage applications. At present, however, the capacity of NIBs is too low for practical applications. Detailed studies of the Na+ storage and charge compensation mechanisms in the anode and cathode are required to inform the rational design of new cathodes suitable for grid-based electrochemical storage applications.
A cheaper and more sustainable alternative to LIBs are sodium-ion batteries (NIBs). Sodium does not suffer such limitations on global reserves, and its abundance means NIBs can be constructed at a lower cost than LIBs. The low production costs make NIBs ideally suited to grid-scale storage applications. At present, however, the capacity of NIBs is too low for practical applications. Detailed studies of the Na+ storage and charge compensation mechanisms in the anode and cathode are required to inform the rational design of new cathodes suitable for grid-based electrochemical storage applications.
Work in the group has thus far focussed on using solid-state NMR paired with DFT calculations to examine the charge storage mechanism of Na+ in NIB cathode materials.1–3 Studies of the charge storage mechanisms in anodes have focussed on using solid-state NMR and pair distribution function (PDF) analysis.4,5 At present, work in the group on NIBs combines local structural investigations—via in situ and ex situ solid-state NMR and ex situ ESR—with bulk structural investigations—such as electrochemical analysis, operando XRD and SQUID magnetometry. Theoretical calculations of NMR shifts and ESR g-tensors is also being used to support these experimental results.
Reference:
1. "Insights into the Nature and Evolution upon Electrochemical Cycling of Planar Defects in the β-NaMnO 2 Na-Ion Battery Cathode: An NMR and First-Principles Density Functional Theory Approach", RJ Clément, DS Middlemiss, ID Seymour, AJ Ilott, CP Grey, Chem. Mater. 2016, 28 (22), 8228–8239, (DOI: 10.1021/acs.chemmater.6b03074)
2. "Structurally Stable Mg-Doped P2-Na2/3 Mn1−y MgyO2 Sodium-Ion Battery Cathodes with High Rate Performance: Insights from Electrochemical, NMR and Diffraction Studies" RJ Clément, J Billaud, A Robert Armstrong, G Singh, T Rojo, PG Bruce, CP Grey, Energy Environ. Sci. 2016, 9 (10), 3240–3251 (DOI: 10.1039/C6EE01750A)
3. "Direct Evidence for High Na + Mobility and High Voltage Structural Processes in P2-Na x [Li y Ni z Mn 1−y−z ]O 2 (x, y, z ≤ 1) Cathodes from Solid-State NMR and DFT Calculations", RJ Clément, J Xu, DS Middlemiss, J Alvarado, C Ma, YS Meng, CP Grey, J. Mater. Chem. A 2017, 5 (8), 4129–4143 (DOI: 10.1039/C6TA09601H)
4. "Mechanistic Insights into Sodium Storage in Hard Carbon Anodes Using Local Structure Probes", JM Stratford, PK Allan, O Pecher, PA Chater, CP Grey, Chem. Commun. 2016, 52 (84), 12430–12433 (DOI: 10.1039/C6CC06990H)
5. "Investigating Sodium Storage Mechanisms in Tin Anodes: A Combined Pair Distribution Function Analysis, Density Functional Theory, and Solid-State NMR Approach", JM Stratford, M Mayo, PK Allan, O Pecher, OJ Borkiewicz, KM Wiaderek, KW Chapman, CJ Pickard, AJ Morris, CP Grey, J. Am. Chem. Soc. 2017, 139 (21), 7273–7286 (DOI: 10.1021/jacs.7b01398)
List of selected publications:
"Investigating Sodium Storage Mechanisms in Tin Anodes: A Combined Pair Distribution Function Analysis, Density Functional Theory, and Solid-State NMR Approach", JM Stratford, M. Mayo, PK Allan, O Pecher, OJ Borkiewicz, KM Wiaderek, KW Chapman, CJ Pickard, AJ Morris and CP Grey, J. Am. Chem. Soc. (2017), ASAP (DOI: 10.1021/jacs.7b01398)
"Mechanistic Insights into Sodium Storage in Hard Carbon Anodes using Local Structure Probes", JM Stratford, PK Allan, O Pecher, PA Chater and CP Grey, Chem. Commun. (2016), 52, 12430 (DOI: 10.1039/c6cc06990h)
"Insights into the Nature and Evolution upon Electrochemical Cycling of Planar Defects in the β-NaMnO2 Na-ion Battery Cathode: An NMR and First-Principles Density Functional Theory Approach", RJ Clément, DS Middlemiss, ID Seymour, AJ Ilott and CP Grey, Chem. Mater. (2016), 28, 8228 (DOI: 10.1021/acs.chemmater.6b03074)
"Sodium Intercalation Mechanism of 3.8 V Class Alluaudite Sodium Iron Sulfate", G Oyama, O Pecher, KJ Griffith, SI Nishimura, R Pigliapochi, CP Grey and A Yamada, Chem. Mater. (2016), 28, 5321 (DOI: 10.1021/acs.chemmater.6b01091)
Lithium-Air
 The lithium-air battery is, in principle, a promising candidate for use as an energy storage system. Theoretically, it can store up to 3,505W.h.kg-1 (approaching an order of magnitude more than a conventional lithium ion battery) based on the reaction (in non-aqueous electrolytes) of lithium and oxygen to form lithium peroxide and including the weight of the reactants. In practice the development of the battery is still at initial stages with operating cells falling short of their promising potential. Among the challenges to be addressed are the identification of stable electrolyte systems, inert and porous cathode materials and efficient catalytic species. These targets can only be realised with a careful analysis of the electrochemical products formed during the operation of the cell.
The lithium-air battery is, in principle, a promising candidate for use as an energy storage system. Theoretically, it can store up to 3,505W.h.kg-1 (approaching an order of magnitude more than a conventional lithium ion battery) based on the reaction (in non-aqueous electrolytes) of lithium and oxygen to form lithium peroxide and including the weight of the reactants. In practice the development of the battery is still at initial stages with operating cells falling short of their promising potential. Among the challenges to be addressed are the identification of stable electrolyte systems, inert and porous cathode materials and efficient catalytic species. These targets can only be realised with a careful analysis of the electrochemical products formed during the operation of the cell.
In our lab we apply multi-nuclear solid state NMR spectroscopy, supported by other techniques such as electron microscopy and X-ray diffraction, to monitor the evolution of electrochemical processes in the battery and gain insight on the performance of various electrolytes, cathode materials and potential catalysts. NMR is a unique and powerful tool in the study of this system as it is sensitive to the entire bulk of the cathode and can detect both crystalline and amorphous materials.
List of selected publications:
"Cycling Li-O2 batteries via LiOH formation and decomposition", T Liu, M Leskes, W Yu, AJ Moore, L Zhou, PM Bayley, G Kim and CP Grey, Science (2015) 350, 530 (DOI: 10.1126/science.aac7730)
"Direct Detection of Discharge Products in Lithium–Oxygen Batteries by Solid-State NMR Spectroscopy", M Leskes, NE Drewett, LJ Hardwick, PG Brice, GR Goward and CP Grey, Angew. Chem. Int. Ed. (2012) 51, 8560 (DOI: 10.1002/anie.201202183)
All-Solid-State Batteries
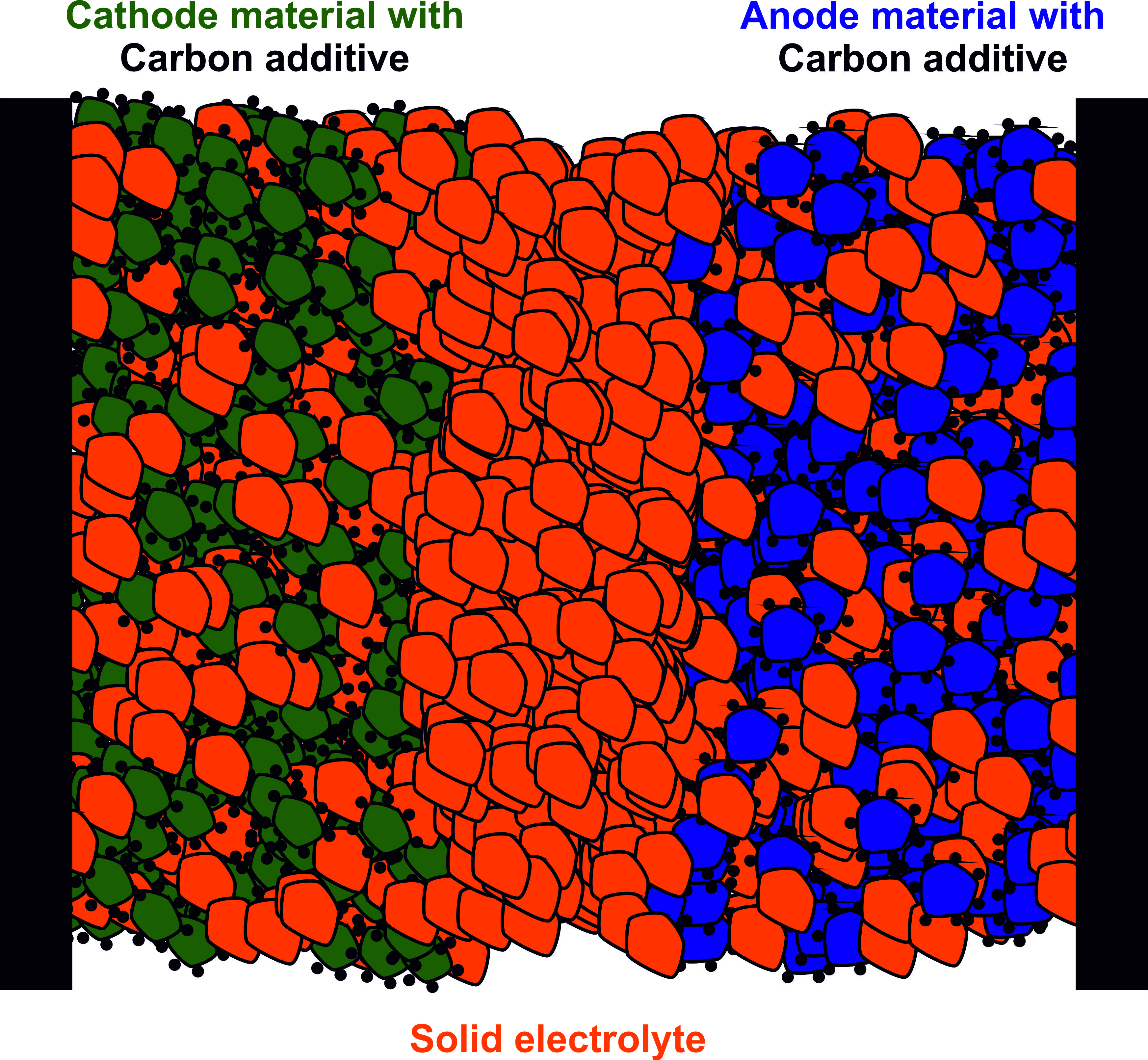 One of the major concerns of current battery technology is their safety. Current batteries often use highly flammable and often toxic liquid electrolytes. In cases of overheating or short-circuits, these liquid electrolytes pose an enormous satefy hazard. One current approach pursued to achieve safer batteries is to go towards all-solid-state batteries (ASSBs), i.e. batteries where not only the electrodes but also the electrolyte are solid materials. This promises safer batteries and opens up possibilities for higher gravimetric and volumetric energy densities. One such possibility is the opportunity to use Li metal as an anode material, if the solid electrolyte is stable and blocks the dendrites which lead to short-circuits in regular batteries.
One of the major concerns of current battery technology is their safety. Current batteries often use highly flammable and often toxic liquid electrolytes. In cases of overheating or short-circuits, these liquid electrolytes pose an enormous satefy hazard. One current approach pursued to achieve safer batteries is to go towards all-solid-state batteries (ASSBs), i.e. batteries where not only the electrodes but also the electrolyte are solid materials. This promises safer batteries and opens up possibilities for higher gravimetric and volumetric energy densities. One such possibility is the opportunity to use Li metal as an anode material, if the solid electrolyte is stable and blocks the dendrites which lead to short-circuits in regular batteries.
Most of the main challenges for ASSBs are related to the interface. While in liquid systems, reaction products can be transported away from the interface, in all-solid systems reaction products stay exactly where they are formed. This can lead to passivation of the interface if it is unstable and unfavourable products are formed (i.e. Li-ion blocking). Additionally, the liquid electrolyte is inherently better in adapting to volume changes of the electrode material during cycling. In ASSBs delamination and contact loss is a big problem, in particular if the electrode material undergoes huge volume changes.
In the group we use for example Magnetic Resonance Imaging (MRI) to image the propagation of dendrites in solid electrolytes, NMR to study decomposition products of interfacial reactions and Pulsed Laser deposition(PLD) and other physical deposition techniques to create model interfaces for further studies.
List of selected publications:
"7Li NMR Chemical Shift Imaging To Detect Microstructural Growth of Lithium in All-Solid-State Batteries", L. E. Marbella, S. Zekoll, J. Kasemchainan, S. P. Emge, P. G. Bruce, C. P. Grey, Chem. Mater. (2019) 31, (8), 2762-2769 (DOI: 10.1021/acs.chemmater.8b04875)
"Interface Instability in LiFePO4–Li3+xP1–xSixO4 All-Solid-State Batteries", M. F. Groh, M. J. Sullivan, M. W. Gaultois, O. Pecher, K. J. Griffith, C. P. Grey, Grey, Chem. Mater. (2019) 30, (17), 5886-5895 (DOI: 10.1021/acs.chemmater.8b01746)
Magnesium Ion Batteries
Multivalent ions such as Mg2+, Ca2+, Al3+, are interesting in the context of rechargeable batteries as they could lead to batteries with increased gravimetric (by weight) and volumetric (by size) capacity. This relatively new field presents many challenges and all components - cathode, anode, electrolyte, casing - require optimised materials.
In collaboration with the Wright group in the Department of Chemistry, we have been investigating the performance and degradation mechanism of new electrolyte systems based on magnesium hexafluorophosphate. We also use 25Mg NMR and DFT to understand reaction mechanisms in positive and negative electrode materials for magnesium-ion batteries.
List of selected publications:
"Insights into the Electrochemical Performances of Bi Anodes for Mg Ion Batteries using 25Mg Solid State NMR Spectroscopy", Z Liu, J Lee, G Xiang, HFJ Glass, EN Keyzer, SE Dutton and CP Grey, Chem. Commun. (2017) 53, 743 (DOI: 10.1039/c6cc08430c)
"A Systematic Study of 25Mg NMR in Paramagnetic Transition Metal Oxides: Applications to Mg-ion Battery Materials", J Lee, ID Seymour, AJ Pell, SE Dutton, and CP Grey, Phys. Chem. Chem. Phys. (2016) 19, 613 (DOI: 10.1039/c6cp06338a)
"Mg(PF6)2-Based Electrolyte Systems: Understanding Electrolyte-Electrode Interactions for the Development of Mg-Ion Batteries", EN Keyzer, HFJ Glass, Z Liu, PM Bayley, SE Dutton, CP Grey and DS Wright, J. Am. Chem. Soc. (2016) 138, 8682 (DOI: 10.1021/jacs.6b04319)
Solid Electrolyte Interphase (SEI)
The formation of a stable SEI is critical to the long term capacity retention of lithium-ion batteries. Lithium is consumed in its formation resulting in irreversible capacity loss, and its growth is correlated with increased cell impedance. We are systematically studying SEI formation on battery materials including silicon-carbon composite electrodes and silicon nanowires.
In addition to NMR, classical and quantum mechanical modelling of molecular and interfacial interactions important for understanding the SEI.
List of selected publications:
"Solid Electrolyte Interphase Growth and Capacity Loss in Silicon Electrodes", AL Michan, G Divitini, AJ Pell, M Leskes, C Ducati and CP Grey, J. Am. Chem. Soc. (2016) 138, 7918 (DOI: 10.1021/jacs.6b02882)
"Fluoroethylene Carbonate and Vinylene Carbonate Reduction: Understanding Lithium-Ion Battery Electrolyte Additives and Solid Electrolyte Interphase Formation", AL Michan, BS Parimalam, M Leskes, RN Kerber, T Yoon, CP Grey and BL Lucht, Chem. Mater. (2016) 28, 8149 (DOI: 10.1021/acs.chemmater.6b02282)
"C-13 Solid State NMR Suggests Unusual Breakdown Products in SEI Formation on Lithium Ion Electrodes", N Leifer, MC Smart, GKS Prakash, L Gonzalez, L Sanchez, KA Smith, P Bhalla, CP Grey, SG Greenbaum, J. Electrochem. Soc. (2011) 158, A471 (DOI: 10.1149/1.3559551)
